Support:Documents:Examples:FDG with Time-varying Rate Constants
FDG Model with Time-varying Rate Constants
Overview
This example demonstrates how to implement the two-tissue compartment model with extended such that K1 and k2 vary in time.
This models the case where there are physiologic changes during the course of the PET scanning. This might happen if tumors are being treated during scanning.
The standard FDG model was described by Phelps and Huang .
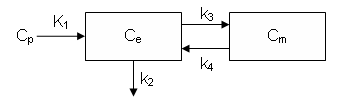
It can also be described by the differential equations:
dCe/dt = K1 Cp - k2 Ce - k3 Ce + k4 Cm
dCm/dt = k3 Ce - k4 Cm
Ce is the tissue concentration of FDG and Cm as the tissue concentration of metabolized FDG (FDG-6-phosphate)
Ce and Cm are interpreted as molar concentrations (Salinas, Muzic and Saidel 2007).
In the standard model. the rate constants K1, k2, k3 and k4 are assumed to be independent of time.
Here we allow K1 and k2 to be linear functions of time:
K1(t) = K10 + a t
k2(t) = K20 + b t
a and b are the derivatives (slopes) of the rate constant with respect to time.
To implement the time-varying rate constants, we create a MATLAB function in a .m file.
By making the function general, the same function can be used for K1 and k2.
function varargout = kinLinearTime(t, c, ncomp, nsens, pName, pValue, pSensIdx, cm, xtra)
% rate constant varies linearly with time: k = k0 + m*t where m = dk/dt
K0Name = pName{1};
dkdtName = pName{2};
K0Value = pValue{1};
dkdtValue = pValue{2};
K0SensIndex = pSensIdx{1};
dkdtSensIndex = pSensIdx{2};
if (nargout > 0), % return effective rate constant
v = K0Value + dkdtValue * t;
if (v < 0), % don't allow negative values for rate constant
varargout{1} = 0;
else
varargout{1} = v;
end
if (nargout > 1), % return dk/dc
dkdc = zeros([1 ncomp]);
varargout{2} = dkdc;
if (nargout > 2), % return dk/dp
dkdp = zeros([1 nsens]);
if (v >= 0),
dkdp(K0SensIndex) = 1;
dkdp(dkdtSensIndex) = t;
end
varargout{3} = dkdp; % dkdp
if (nargout > 3),
ddkdpdc = zeros([ncomp nsens]);
varargout{4} = ddkdpdc; % ddkdpdc
end
end
end
end
return
This function is pretty straightforward. The emphasis of our description here is on the mathematical aspects.
The first output argument varargout{1} holds the value of the rate constant specified at the current time t.
This value is first calculated and stored in the variable v.
v = K0Value + dkdtValue * t
k0 is the rate constant at time zero (t=0) and dkdtValue is the increase in the rate constant per unit time (time-derivative or slope). k0Value and dkdtValue are the MATLAB variables that hold the values of these parameters. The value of v is checked to see if it is negative and, if it is, the value zero is used instead since negative values for rate constants are non-physiologic.
The line
varargout{2} = dkdc;
returns the derivative of the rate constant with respect to all the compartment concentrations (a vector). In this case, the rate constant is not dependent on the concentrations so the derivatives are all zeros.
The lines
dkdp(K0SensIndex) = 1; dkdp(dkdtSensIndex) = t;
calculate the derivatives of the rate constant with respect to the model parameters k0 (the initial value of the rate constant) and dkdt (the increase in k per unit time). Since a model can have other parameters besides these, the function stores these derivatives in the appropriate element of the derivative vector. Derivatives of this rate constant with respect to all other parameters are zeros.
The line
varargout{4} = ddkdpdc; % ddkdpdc
returns the values of the mixed derivatives (derivative of rate constant with respect to concentrations and with respect to time), which, in this case are all zeros.
The function is stored in kinLinearTime.m in the comkat examples folder.
This function is called to evaluate both K1 and k2.
Implementing the Compartment Model
Create a new model
First define some MATLAB variables for clarity and to facilitate exploring what happens if values are changed
K10 = 0.15; % K1 = K10 + dK1dt * t dK1dt = -K10 * 0.5 / 60; % 50% decrease per 60 minutes k20 = 0.325; % k2 = k20 + dk2dt * t dk2dt = -k20 * 0.5 / 60; % 50% decrease per 60 minutes k3 = 0.05; k4 = 0.007;
Next, create a compartment model object and define the parameters within the model object
% create empty compartmentModel object cm = compartmentModel; % define the parameters cm = addParameter(cm, 'K10', K10); % 1/min cm = addParameter(cm, 'k20', k20); % 1/min cm = addParameter(cm, 'k3', k3); % 1/min cm = addParameter(cm, 'k4', k4); % 1/min cm = addParameter(cm, 'dK1dt', dK1dt); % 1/min/min cm = addParameter(cm, 'dk2dt', dk2dt); % 1/min/min cm = addParameter(cm, 'sa', 1); % specific activity of injection cm = addParameter(cm, 'dk', log(2)/109.8); % F-18 radioactive decay cm = addParameter(cm, 'PV', 1); % (none)
Now, define the model compartments. The first two are Ce and Cm as described above and Junk is a "sink" that collects the FDG that is cleared by the venous circulation.
cm = addCompartment(cm, 'Ce'); cm = addCompartment(cm, 'Cm'); cm = addCompartment(cm, 'Junk');
For the plasma concentration time-course of FDG or input function, we'll use the
Feng input
which is an analytic expression. Since piecewise polynomials (linear interpolation, cubic splines, etc...) are widely used in COMKAT and their implementation has been optimized,
here we construct a cubic spline representation of the input function by sampling the Feng input.
x0=[2 0.5 16.000 2.188 2.081 -7.5 -10 -0.01043]; % Feng parameters t = [0:.1:3 3.5 4 4.5 5:1:10 12 15:5:(expDuration+5)]; Cp = fengInput(x0, t); ppCp = spline(t, Cp); cm = addInput(cm, 'Cp', 'sa', 'dk', 'ppval', ppCp); % Cp has units of pmol/ml
The PET scanner measurement is assumed to represent the sum of FDG and FDG-6-Phosphate so the model output is calculated as the sum of the Ce and Cm compartments
Wlist = {...
'Ce', 'PV';
'Cm', 'PV'};
Xlist = {};
cm = addOutput(cm, 'PET', Wlist, Xlist);
Next define the scan frames as the start and stop time of each image in the dynamic sequence.
ttt=[ ones(12,1)*10/60; ... % 10 sec
ones(10,1)*0.5; ... % 0.5 min
ones(10,1)*2;... % 2 min
ones(10,1)*5;... % 5 min
ones(4,1)*10]; % 10 min
scant=[[0;cumsum(ttt(1:(length(ttt)-1)))] cumsum(ttt)];
cm = set(cm, 'ScanTime', scant);
Note the acquisition begins with 12 frames of 10 sec duration, then has 10 frames of 0.5 minute duration, etc... The cumsum() function calculates the cumulative summation. Thus, the first column of scant holds the scan start times and the second column holds the scan end times. These are expressed in minutes:
scant =
0 0.1667
0.1667 0.3333
0.3333 0.5000
0.5000 0.6667
0.6667 0.8333
0.8333 1.0000
1.0000 1.1667
1.1667 1.3333
1.3333 1.5000
1.5000 1.6667
1.6667 1.8333
1.8333 2.0000
...
% make copy of model thus far cmRef = cm;
% define rate constants cm = addLink(cm, 'LSpecial', 'Cp', 'Ce', 'K1', 'kinLinearTime', {'K10', 'dK1dt'}); cm = addLink(cm, 'KSpecial', 'Ce', 'Junk', 'k2', 'kinLinearTime', {'k20', 'dk2dt'}); cm = addLink(cm, 'K', 'Ce', 'Cm', 'k3'); if strcmp(modelConfig, '4k'),
cm = addLink(cm, 'K', 'Cm', 'Ce', 'k4');
end
% define reference model with time-invariant rate constants cmRef = addLink(cmRef, 'L', 'Cp', 'Ce', 'K10'); cmRef = addLink(cmRef, 'K', 'Ce', 'Junk', 'k20'); cmRef = addLink(cmRef, 'K', 'Ce', 'Cm', 'k3'); if strcmp(modelConfig, '4k'),
cmRef = addLink(cmRef, 'K', 'Cm', 'Ce', 'k4');
end
% solve both models and compare outputs
[PET, PETIndex] = solve(cm);
[PETRef, PETRefIndex] = solve(cmRef);
tmid = (PET(:,1) + PET(:,2)) / 2;
figure clf axK1k2 = axes('position', [0.2 0.75 0.7 0.2]); phK = plot(axK1k2, tmid, K10 + dK1dt * tmid, 'b', tmid, k20 + dk2dt * tmid, 'r', ...
tmid, K10 + 0 * tmid, 'b:', tmid, k20 + 0 * tmid, 'r:');
set(phK, 'LineWidth', 1.5); ax = axis; ax(3) = 0; ax(4) = 0.5; axis(ax); ylabel('Rate Const. (1/min)'); lh = legend('K1', 'k2', 'K1', 'k2', 'Location', 'SouthEast'); set(lh, 'fontsize', 8);
axPET = axes('position', [0.2 0.2 0.7 0.4]); phPET = plot(axPET, tmid, PET(:,3), 'b', tmid, PETRef(:,3), 'b:'); set(phPET, 'LineWidth', 1.5); ax = axis; ax(3) = 0; axis(ax); xlabel('Time (Minutes)'); ylabel('Activity (uCi/ml)'); legend('Time-varying', 'Reference', 'Location', 'SouthEast');
% What would be the value of rate constants estimated while neglecting the time-variation?
% Fit the time-invariant model to the time-varying model data.
data = PET(:,3);
cmRef = set(cmRef, 'ExperimentalData', data);
pinit = [0.07; 0.1; 0.04; 0.005];
plb = [0.01; 0.001; 0.001; 0.0001];
pub = [0.5; 0.5; 0.2; 0.01];
if strcmp(modelConfig, '4k'),
cmRef = addSensitivity(cmRef, 'K10', 'k20', 'k3', 'k4');
else
cmRef = addSensitivity(cmRef, 'K10', 'k20', 'k3'); pinit(4) = []; plb(4) = []; pub(4) = [];
end
[pfit, modelFit] = fit(cmRef, pinit, plb, pub);
figure phFit = plot(tmid, data, 'bo', tmid, modelFit, 'b:'); set(phFit, 'LineWidth', 1.5); ax = axis; ax(3) = 0; axis(ax); xlabel('Time (Minutes)'); ylabel('Activity (uCi/ml)');
KiRange = [K10*k3/(k20 + k3); (K10 + dK1dt)*k3/(k20 + k3); K10*k3/((k20 + dk2dt) + k3); (K10 + dK1dt)*k3/((k20 + dk2dt) + k3)]; KiInit = K10*k3/(k20 + k3); if strcmp(modelConfig, '4k'),
c = {'Parm.', 'True', 'Est', 'vs. Init', 'Units'; ...
'K1', sprintf('%1.3f -> %1.3f', K10, K10 + dK1dt * PET(end,2)), sprintf('%1.3f', pfit(1)), sprintf('%1.1f%%', 100*(pfit(1)-K10)/K10), '1/min';
'k2', sprintf('%1.3f -> %1.3f', k20, k20 + dk2dt * PET(end,2)), sprintf('%1.3f', pfit(2)), sprintf('%1.1f%%', 100*(pfit(2)-k20)/k20), '1/min';
'k3', sprintf('%1.3f', k3), sprintf('%1.3f', pfit(3)), sprintf('%1.1f%%', 100*(pfit(3)-k3)/k3), '1/min';
'k4', sprintf('%1.3f', k4), sprintf('%1.3f', pfit(4)), sprintf('%1.1f%%', 100*(pfit(4)-k4)/k4), '1/min';
'Ki', sprintf('%1.3f - %1.3f', min(KiRange), max(KiRange)), sprintf('%1.3f', pfit(1)*pfit(3)/(pfit(2)+pfit(3))), sprintf('%1.1f%%', 100*(pfit(1)*pfit(3)/(pfit(2)+pfit(3))-KiInit)/KiInit), '1/min';
};
else
c = {'Parm.', 'True', 'Est', 'vs. Init', 'Units'; ...
'K1', sprintf('%1.3f -> %1.3f', K10, K10 + dK1dt * PET(end,2)), sprintf('%1.3f', pfit(1)), sprintf('%1.1f%%', 100*(pfit(1)-K10)/K10), '1/min';
'k2', sprintf('%1.3f -> %1.3f', k20, k20 + dk2dt * PET(end,2)), sprintf('%1.3f', pfit(2)), sprintf('%1.1f%%', 100*(pfit(2)-k20)/k20), '1/min';
'k3', sprintf('%1.3f', k3), sprintf('%1.3f', pfit(3)), sprintf('%1.1f%%', 100*(pfit(3)-k3)/k3), '1/min';
'Ki', sprintf('%1.3f - %1.3f', min(KiRange), max(KiRange)), sprintf('%1.3f', pfit(1)*pfit(3)/(pfit(2)+pfit(3))), sprintf('%1.1f%%', 100*(pfit(1)*pfit(3)/(pfit(2)+pfit(3))-KiInit)/KiInit), '1/min';
};
end
table(c, [0.4, 0.3] ); legend('Time-varying Data', 'Time-invariant Fit', 'Location', 'SouthEast');
First create an new model stored in variable bfcm (blood flow compartment model) and add compartments. The J compartment is not shown in the figure above but is needed to accept the flux from the k2 arrow.
% create new, empty model bfcm = compartmentModel; % add compartments bfcm = addCompartment(bfcm, 'CT'); bfcm = addCompartment(bfcm, 'J');
Specify the input
The specific activity (ratio of activity to molar concentration) is an exponentially decaying function S(t) = S0 exp(-0.341 t) where t is expressed in units of minutes and 0.341 = ln(2)/half-life of 15O and S0 is the specific activity at time t = 0.
% define the initial specific activity and decay constant sa0 = 1; % specific activity at t=0 dk = 0.341; % O-15 decay constant
Next specify the input functions Cp and Ca for the compartment model. fin is the name of the function that implements Feng input. pCp and pCa are parameter vectors that hold the values of p1, p2, ... p6 for Cp and Ca.
bfcm = addInput(bfcm, 'Cp', sa0, dk, 'fin', 'pCp'); bfcm = addParameter(bfcm, 'pCp', 2*[851.1 21.88 20.81 -4.134 -0.1191 -0.01043 .5]); bfcm = addInput(bfcm, 'Ca', sa0, dk, 'fin', 'pCa'); bfcm = addParameter(bfcm, 'pCa', [851.1 21.88 20.81 -4.134 -0.1191 -0.01043 .5]);
Define the connections (arrows) between the compartment and input. The link type 'L' indicates linear kinetics from an input to a compartment and type 'K' indicates linear kinetics from one compartment to another.
% connect compartments and inputs bfcm = addLink(bfcm, 'L', 'Cp', 'CT', 'K1'); bfcm = addLink(bfcm, 'K', 'CT', 'J', 'k2'); % define values for K1, k2 ... bfcm = addParameter(bfcm, 'K1', 'F'); bfcm = addParameter(bfcm, 'k2', 'K1/lambda'); bfcm = addParameter(bfcm, 'F', 0.5); bfcm = addParameter(bfcm, 'lambda', 1.0); bfcm = addParameter(bfcm, 'Fv', 0.05);
Define tissue and blood outputs
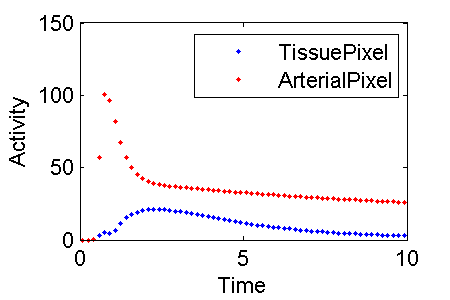
Define two model outputs. The output called TissuePixel predicts the activity in a tissue pixel and the output called ArterialPixel predicts the activity that would be observed in a pixel in the aorta. TissuePixel corresponds to CTS + FvCa whereas ArterialPixel is 100% Ca.
% define the activity concentration in tissue pixel
bfcm = addOutput(bfcm, 'TissuePixel', {'CT', '1'}, {'Ca', 'Fv'});
% define the activity concentration in arterial blood pixel
bfcm = addOutput(bfcm, 'ArterialPixel', {'CT', '0'}, {'Ca', '1'});
Note that COMKAT even accounts for the time-averaging of concentrations over the durations of the scan frames. For simplicity here a sequence of contiguous 10-second frames is used. (Frame start times 0, 10, 20, ... 590 sec and frame end times 10, 20, 30, ... 600 sec).
% specify scan frame times bfcm = set(bfcm, 'ScanTime', [[0:10:590]' [10:10:600]']/60); % division by 60 converts sec to min
Solve for the model output
Finally, tell COMKAT to solve the model to obtain simulation data that will be used in fitting.
[PET, PETIndex, Output, OutputIndex] = solve(bfcm);
% plot activity in tissue PET(:,3) and blood PET(:,4)
midTime = (PET(:,1) + PET(:,2)) / 2;
plot(midTime, PET(:,3), 'b-', midTime, PET(:,4), 'r.')
legend(PETIndex{3}, PETIndex{4})
% make the figure for this wiki
set(1,'PaperPosition',[0.25 2.5 3 2])
print -dpng c:\temp\ExampleFigureBloodFlowInputModelDataToFit.png
Note, different rows of PET correspond to different frames. The columns of PET hold various quantities as indicated in PETIndex. That is, the contents of column i are described in PETIndex{i}.
Fit Data
Modify the COMKAT model a bit so that it can be used to fit data created above.
Specify the data to fit
Just use the result from above.
data = PET(:, [3 4]); bfcm = set(bfcm, 'ExperimentalData', data);
Specify parameters to estimate along with their initial guesses and range of feasible values.
% specify parameters to estimate bfcm = addSensitivity(bfcm, 'pCp', 'F'); % define initial guess and lower and upper bounds pCpInit = 0.9*2*[851.1 21.88 20.81 -4.134 -0.1191 -0.01043 .5]; FInit = 0.3; pinit = [pCpInit FInit]; lb = [2*[85 2 2 -8 -3 -0.1 .05] 0.1]; ub = [2*[2000 100 200 -1 -0.01 -0.001 2] 1.];
Note the values of the initial guesses for the input function parameters are 0.9 times the true values. (We're are not cheating by starting with the correct values.)
Do the actual fitting
Fit the data to estimate values of the parameters
[pEst, modelFit] = fit(bfcm, pInit, lb,ub);
COMKAT makes fitting easy!
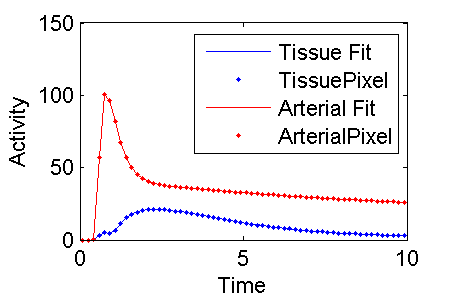
Plot the results and make the figure below for this wiki
plot(midTime, modelFit(:,1), 'b', midTime, PET(:,3), 'b.', midTime, modelFit(:,2), 'r', midTime, PET(:,4), 'r.')
legend('Tissue Fit', PETIndex{3}, 'Arterial Fit', PETIndex{4})
xlabel('Time')
ylabel('Activity')
print -dpng c:\temp\ExampleFigureBloodFlowInputModelFit.png
Ta Da
It looks like a pretty good fit.